|
|
|
Are Nickel-Metal Hydride Batteries Superior to Sealed Lead-Acid in Light Electric Vehicle Applications? |
|||||||||||||||||
 |
|||||||||||||||||
|
By Brad Duncan Table of Contents 1. Question being addressed 7a. Mechanical construction 8. Experiment: Discharge testing SLA and NiMH batteries 8a. Testing methodology 9. Field Trial: SLA vs. NiMH in an Electric Bicycle application 9a. Real-world vs. bench testing 10. Conclusions Table: Comparison of electrical characteristics 1. Question being addressed Are Nickel-metal Hydride batteries superior to Sealed Lead-acid in light electric vehicle applications? 2. Hypothesis/Background I intend to determine if Nickel-metal Hydride (NiMH) is a superior battery chemistry to the older Sealed Lead-acid (SLA) type for light electric vehicles used for personal transportation. I will determine this through research, measurement and observation. My experiment will involve comparing both battery types in a test fixture and in an electric bicycle application. In contrast to conventional cars, Light Electric Vehicles (LEVs) offer dramatically reduced energy consumption. Many of our errands and trips are less than 10 miles, which is within the range of most LEVs. LEVs range in size from electric scooters up to one-person cars. For our comparison we will be using an electric bicycle. Electric bikes are everyday bicycles with an added battery-powered electric motor. They allow for human input, which can extend the range provided by motor assist, 3. Prevalence of LEVs Sales of LEVs have increased anywhere from 40 to 200% annually over the last three or four years in the United States. They can now be purchased at most mass retail chains, like Target or Costco 4. Types of LEVs Electric Scooterswere once thought of strictly as a mobility aid, but now are being embraced by those who appreciate and enjoy the riding experience and the efficiency of them. Electric Bicycles are perhaps the most practical LEV because they allow sustained human input and thus extended range. Some people build them from scratch, but it is much more common that hobbyists and experimenters buy them and modify the motors, drive systems and batteries for additional speed or range. 5. Battery chemistries used in LEVs Electric bikes and scooters are usually powered by Sealed Lead Acid (SLA) or Nickel Metal Hydride (NiMH) batteries in voltages ranging from 12-48 volts. 6. Why I selected NiMH and SLA to compare SLA is an economical and traditional battery chemistry for electric bikes and vehicles. NiMH has emerged as a lighter weight alternative with proven reliability and charging systems. Lithium Ion batteries such as those used in laptops require more complex charging systems and require careful handling and use. Zinc composition batteries are showing great promise but are less commonly available in the amp-hour ratings and price points required for LEV applications. 7. Comparing SLA and NiMH in LEVs In this section I will research and compare various characteristics that contribute to the usability of SLA and NiMH batteries in LEVs. 7a. Mechanical construction The Mechanical construction of a battery contributes directly to the reliability and integrity of the pack. This is important because the pack is subject to vibration and shock that can cause early failures. The SLA battery is fully self-contained in a plastic case. The individual cells are held in place inside the case and connected internally. Internal connection failures are almost unheard of. A 36 volt pack will require three 12 volt SLAs. Only two external connections are required to form a 36 volt pack. In contrast, a 36 volt NiMH pack is composed of thirty individual cylindrical cells. Excluding the primary positive and negative terminals, there are 28 separate connections between batteries, each requiring two solder joints. There are also 30 cells compared to 18 in an SLA pack, so there is more potential for electrical failure as well. Winner: The SLA is mechanically far less complex and thus less subject to mechanical failure. The NiMH pack employs more individual electrical cells, each of which is subject to early failure and faulty connections. 7b. Charging Battery capacity (C) is the amount of current in amps that the battery can supply for one hour. In our tests, we are using an 8 Amp Hour (Ah) SLA, and a 9 Ah NiMH pack. Batteries can only be safely charged at a fraction of C. SLA batteries can be safely charged at .2C NiMH batteries can be safely charged at .5C However, it’s not that simple. The NiMH pack cannot be charged when it is hot from being used. It must be cooled before charging since temperature is one factor used by the battery charger to determine when to shut off. Also, due to the complexity of the NiMH charging algorithm, NiMH chargers are significantly more expensive. Winner: No clear advantage 7c. Discharging (use) SLA batteries cannot be left in a discharged state or sulfation will begin to occur within a few days. NiMH batteries can be left in various states of charge with no detrimental effect. However, NiMH batteries are subject to an effect known as cell reversal, where small differences in voltages of individual cells can result in negative polarity being applied to the weaker cell, causing permanent damage. The maximum depth of discharge for NiMH batteries is 1V per cell, or 30V total, compared to the SLA at 1.75V per cell or 31.5V total. Therefore, the NiMH pack can be used for slightly longer. Both battery chemistries are rated at a maximum discharge current of 5C, or 40A for the SLA pack and 45A for the NiMH pack. Winner: NiMH. Both batteries have mostly equal characteristics, however the NiMH can be discharged slightly further. 7d. Thermal (during use) Both battery chemistries can operate over a range of -20°C to 60°C with limitations.
7e. Thermal (during charging)
7f. Thermal (duty cycle)
Winner: SLA battery. The NiMH battery must be allowed to cool between charging and use or vice versa. This requires a mandatory delay which could diminish usefulness in commuting situations. 7g. Weight considerations Accurately calculating the effect of variations in weight on the top speed of a bicycle, either human-powered or motorized, involves some very detailed calculations. Modeling these factors is beyond the scope of this report, but an accurate and complete online calculator especially created for recumbent bicycles exists at this site: http://www.kreuzotter.de/english/eindex.htm. The following input parameters were used to obtain the top speed: Bicycle weight: 85 lbs Top speed (NiMH): 18.1 mph Winner: No clear winner. Note: in many applications, SLA batteries outweigh NiMH by a greater margin, especially at higher amp-hour ratings. For lighter LEVs in the 35-50 lb range, the speed difference would be more dramatic. 7h. Storage Self-discharge is voltage loss over time. SLAs have a very low self-discharge rate, about 5% per month. NiMH batteries loose far more, about 30% per month. NiMH batteries can be stored at any state of charge without damage. However, SLAs are subject to sulfation, a corrosion of the battery plates if left in a discharged state. This is the single biggest cause of battery damage in LEVs. Winner: NiMH. You can always top off a charge, but sulfation is usually irreversible. 7i. Economic Our SLA pack cost $20 per 12V battery for a total cost of $60. The charger was an additional $40. Total cost for pack and charger: $100 The exact NiMH pack is no longer available, but a comparable pack can be purchased for $300. The charger is more sophisticated and costs $130. Total cost: $430. Winner: SLA pack and charger costs 75% less to purchase than the NiMH. 7j. Longevity The SLA battery can be recharged 200 to 300 times. The NiMH battery can be recharged 300 to 500 times, but will be at the lower end of the scale if fully discharged each time. Since electric vehicle applications demand frequent full discharge to obtain maximum range, the advantage is negated. Winner: No clear winner. 7k. Environmental The lead-acid battery is easily recycled. In the USA, 98% of all lead-acid batteries are recycled. They can be turned in at any automotive service center. In comparison, only one in six households in North America recycles other battery chemistries. Unlike nickel-cadmium cells, nickel-metal-hydride is considered environmentally friendly. However, if ten or more batteries are accumulated, the user should consider disposing of these packs in a secure waste landfill. Winner: SLA batteries for ease of recycling and reclamation of material. 8. Experiment: Discharge testing SLA and NiMH batteries 8a. Testing methodology In order to observe the ability of each battery to provide sustained current over time, I used a resistive load and took voltage measurements at fixed intervals. This was repeated until each battery reached its maximum depth of discharge. 8b. Construction of an air-cooled load resistive load Through the use of an ammeter temporarily fixed to the electric vehicle, I observed that current drain varied between 0 and 30 amperes under normal operating conditions. I chose a continuous 8 amp load because it was close to the battery’s 1C values and could be obtained using available wire-wound resistors. 8c. Safety concerns Whenever working with batteries capable of delivering high currents, it is important to put a fuse in the circuit. I used a 15A fuse which would blow immediately if short circuited. Always verify all connections before applying power. 8d. Selecting the load resistance, power calculation and heat-sinking For the load, I bought 6, 30 ohm, 50 watt wire-wound resistors. Each bank of two in parallel provides an equivalent resistance of 15 ohms. At a nominal voltage of 36 volts, 2.4 amps go through each branch (times 3 = ~7 amps). Power is 86 watts (P=I*E), which is at the high end of the operating range (100 watts) for the two resistors. When used this way, heat sinking is required. Each bank of two resistors was mounted on a heat sink with silicon grease used to help conduct heat. 8e. Use of forced air cooling Even with heat sinks, the temperature could get hot enough to cause burns or melt the apparatus. To insure it operated within safe limits, a cooling fan was attached. 8f. Performing the discharge tests Each battery was freshly charged and connected to the load device in series with an ammeter. A voltmeter was connected across the load terminals. At time zero, the load was switched on and an initial voltage reading was taken. At five minute intervals, the voltage was measured. This was repeated until the maximum depth of discharge voltage was reached. 8g. Test results |
|||||||||||||||||
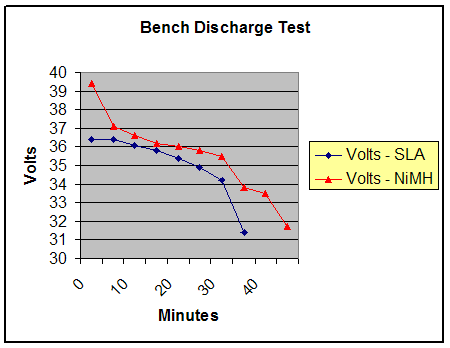 |
|||||||||||||||||
|
The NiMH battery had a higher initial voltage under load and throughout the measurements. It also maintained a usable voltage range for a greater duration than the SLA. The SLA battery began to decline more rapidly than the NiMH, showing a steeper decline, which would equate to less notice to the rider in actual operation. Winner: NiMH battery had more staying power in bench testing. 9. Field Trial: SLA vs. NiMH in an electric bicycle application 9a. Real-world vs. bench testing In actual use, current drain may vary between 0 and 30 amps. To see how each battery holds up in actual use, a discharge test based on a typical on-road course was designed. 9b. The test vehicle The test vehicle was a Compact Long Wheel-Base recumbent bicycle adapted to use a brushless DC electric motor running on 36 volts. A space below the seat allowed interchanging the SLA and NiMH batteries. 9c. Designing the trial A course of approximately 1.5 miles was selected that had a mixture of hills and level ground to simulate typical use. During the course, full throttle was applied on all uphill and level sections. Downhill, the motor and battery were allowed to rest, like in normal use. Since the motor is an assist to pedaling, constant, normal human input was applied whenever the bike was under power. An average speed of 18-22 MPH was maintained during the trials. In addition to motor assist, normal gear changes were used to obtain the target speed when climbing hills or on level terrain. My father, the owner of the bicycle performed the trials. His experience and stamina helped to obtain consistent results. 9d. Taking measurements A digital voltmeter was attached to the bicycle frame. At the beginning of each “lap” was a short but steep hill that would put the battery under full load. Just before reaching the top of this hill, the voltage was noted. Upon cresting the hill, the motor was disengaged. Five seconds later, a second measurement was taken. Both measurements were then recorded into a digital voice recorder. 9e. Results |
|||||||||||||||||
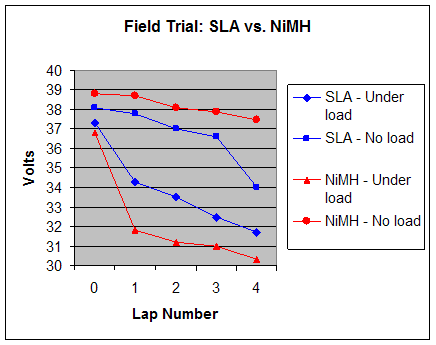 |
|||||||||||||||||
|
As in the bench test, the NiMH battery had a higher initial voltage. Under load was where the differences became visible. The NiMH battery could not sustain it’s voltage under the load typical of normal vehicle operation, which was closer to 30A rather than the 7-8A drawn during bench testing. At this point, more input would be required from the operator to maintain the same speed. Upon cresting the hill, the NiMH battery rebounded to a value closer to its initial voltage, and under more moderate loads provided a higher sustained speed. The SLA battery, despite a lower starting voltage maintained higher potential under full load. It also did not drop as far under full load. Otherwise, its performance was similar to the NiMH until its characteristic steep decline in voltage as seen in bench testing occurred. Again, the NiMH outlasted the SLA in total usable time. Winner: NiMH by a minor margin. It lasted longer overall, but did not hold up as well under load. The SLA also lost points for dropping off steeply at the end. 10. Conclusions As outlined in this report, there are numerous factors to consider when comparing specific battery chemistries for use in LEVs. Either battery can be obtained in the size and amp-hour rating suitable to provide acceptable speed and range for practical operation. Bench testing and field trials demonstrate that although NiMH will provide slightly higher maximum speeds under nominal loads, the SLA battery yields acceptable performance and actually performs better on extreme grades. One then needs to look at their own objectives and goals for owning and operating an LEV. If ultimate economy is the goal, the SLA is certainly less expensive to purchase and replace (which all batteries eventually need). If top speed and lightest weight is desired, the NiMH pack is the clear winner. In our tests, the batteries were similar in weight, but when higher capacity SLA batteries are used, the weight difference becomes a factor worth considering. For the operator who wants the highest degree of reliability, the SLA has the obvious advantage. It not only has substantially less electrical connections, the total number of individual cells is almost halved compared to the NiMH. NiMH batteries also have a disadvantage in thermal characteristics. They need to cool before being recharged and require a more sophisticated and expensive charger to avoid overcharging. SLA on the other hand, can be damaged if not charged after use. Perhaps the most compelling argument for the SLA battery chemistry is in its environmental advantages. Lead recycling is commonly available, and a very large percentage of the battery by weight is reclaimed. Although NiMH batteries do not contain the toxic metal cadmium like their close cousins, access to recycling facilities for the average consumer is far less common and they are much more likely to end up in a landfill. For the time being, lead is still a practical choice in Light Electric Vehicles. 11. References Cadex Electronics - “Charging Algorithms of Sealed Lead-acid Batteries” Panasonic, Inc – “Charge Methods for Nickel-Metal Hydride Batteries” Cadex Electronics – “Battery University” Isidor Buchmann – “Batteries in a Portable World - A Handbook on Rechargeable Batteries for Non-engineers” Walter Zorn - A Speed and Power Calculator for Recumbent Bicycles |
|||||||||||||||||
|
Table 3: Comparison of electrical characteristics |
|||||||||||||||||
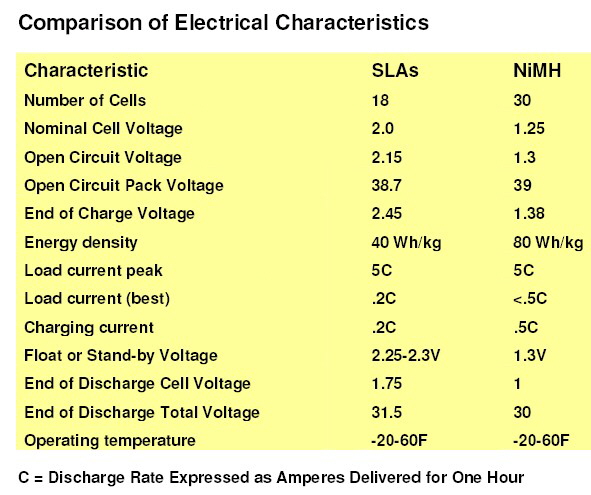 |
|
B. Duncan 12/2007 |
|
[Home] [E-Bikes & Scooters] [My E-bikes] [Scooterama] [Battery Tech] [LEV Info] [I'm Car Crazy] [Advice & Opinion] [My Photography] [Tendon Pain?] [About Me] |